Fridge, Freeze or Fix: What to Do if You Canã¢â‚¬â„¢t Analyze Your Cells by Flow Cytometry Immediately
Prepare Now? Fix Later? - Considerations for the Employ of Paraformaldehyde Fixation in Flow Cytometry
- Staining intracellular targets (e.thousand. − intracellular cytokine staining, phosphorylation targets) - the cells demand to be stock-still prior to the permeabilization of the cells.
- Convenience − for scheduling purposes, you need to fix the samples in the centre of an experiment so you can keep to clarify them later.
- Disinfection − when you're working with infectious samples (i.e. HIV-infected patient samples), it's a routine procedure to pre-fix the sample prior to assay for sanitation purposes.
- Timepoint − when you're looking at a progressive biological process over a fourth dimension-course (east.g.- phosphorylation of Protein A, X hours afterward stimulation Y), yous'll desire to "freeze" all processes using fixation so you lot get a more accurate snapshot of what's going on at different timepoints.
- It's Sat night - your buddy Chad is having a birthday bash at the Legendary bar downtown starting at 8pm. Information technology's 8:30 already, you're in the lab, and have simply finished harvesting your samples. I hateful… you have no choice, correct?
-
- What concentration of fixative should I use for my experiment?
- When in your staining procedure should you fix your sample in the duration of your protocol?
- Ultimately, how tin these decisions potentially bear upon my flow staining?
- What concentration of fixative should I use for my experiment?
In this blog, we'll explore these questions related to fixation, and perchance some nutrient for thought for your next menstruum cytometry experiment when you see a fixation dilemma.
A solution ranging from 1-four% PFA is typically used for fixation of samples for flow cytometry. In the instance of sanitizing infectious samples, concentrations as low every bit 0.37% can effectively disinfect samples from HIV-infected patients 1 . Incubation for up to 45-hr with 1% PFA, and 15-twenty minutes with iv% PFA (east.m. BioLegend'southward buffer) is sufficient to fully fix the cells, and the cells can either be used for downstream processing (permeabilization for intracellular targets) or stored for future analysis at this stage.
During a routine flow cytometry staining procedure that involves surface marker staining, there are several steps where you lot can determine to fix your samples - fix after surface marker staining, or prepare before .
In some instances, the conclusion for when you tin set up is... fixed. For instance, if you're analyzing phospho-targets, bounden of sure surface antigens past antibodies tin change intracellular signaling pathways shortly afterward contact, thus leading to an artifact in your phosphorylation results. In which case, you should fix the cells before the addition of the surface antibodies (which is why our recommended protocol to use for our True-Phos™ Perm Buffer to analyze intracellular phosphorylation targets is designed so that the fixation step precedes other procedures). Each has its own benefits and drawbacks, but here's a couple of things to watch out for in each instance.
Fixation BEFORE staining - epitope amending:
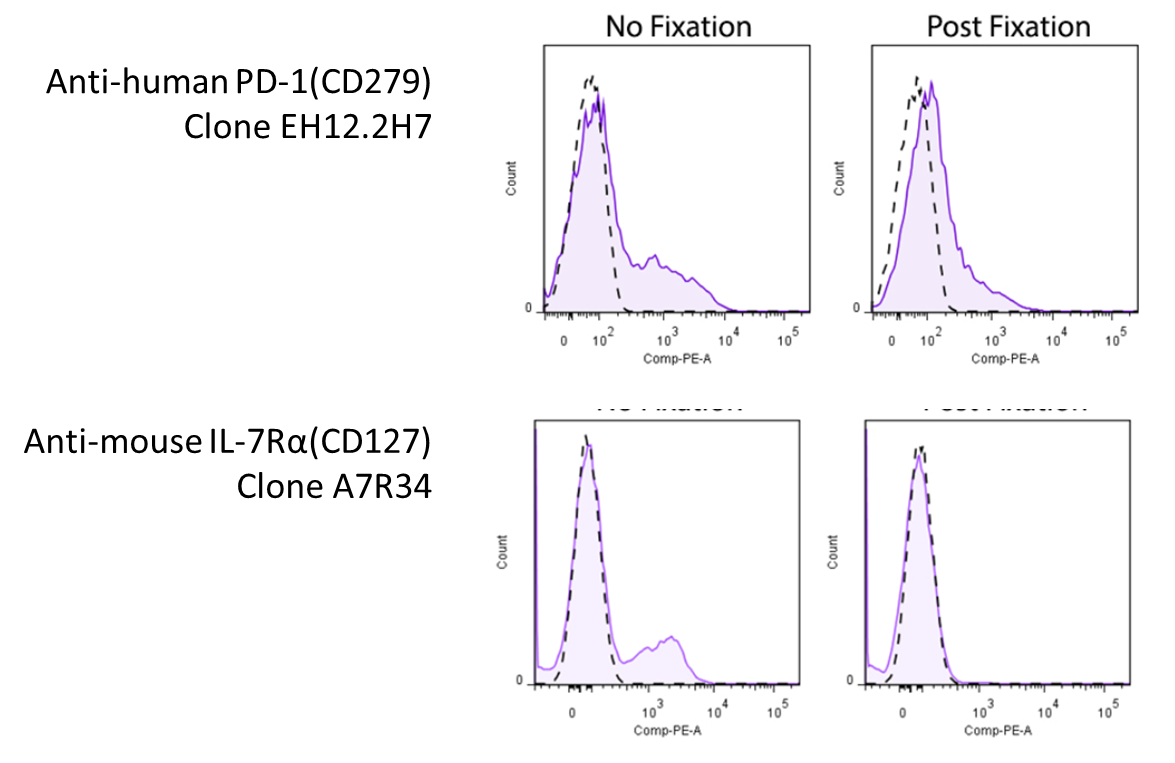
Representative plots for target cells stained with (Mail service-Fixation) or without 4% PFA fixation.
Since this effect depends on the clone of antibody yous're using, you volition want to expect around to see whether there's whatever information pertaining to the antibody you're using. Yet, yous might be in luck! We've compiled a ready of information and data (where available) on whether a particular antibody'southward binding is affected by iv% PFA fixation, which can be found on our Fixation webpage. For clones not listed on our site, you may want to consult the literature.
Fixation Later staining - fluorophore stability
To combat the consequence of tandem breakup upon fixation, BioLegend has developed the FluoroFix™ Buffer - a PFA-based fixative buffer specially formulated to mitigate signal quenching by sensitive fluorophores.
Exercise you take any other questions regarding fixation procedures for your flow cytometry piece of work? Any additional tips and considerations to add here? Allow us know at tech@biolegend.com!
Source: https://www.biolegend.com/en-us/blog/fix-now-fix-later-considerations-for-the-use-of-paraformaldehyde-fixation-in-flow-cytometry
0 Response to "Fridge, Freeze or Fix: What to Do if You Canã¢â‚¬â„¢t Analyze Your Cells by Flow Cytometry Immediately"
Postar um comentário